The amount of a particular substance can be determined by measuring the volume of reactant solutions, provided that you know the stoichiometry of the reaction and the concentration and volume of one of them.
Example of acid-base neutralization
What volume of 0.375 M HCl is needed to react completely with 35.0 mL of 0.250 M solution of Ca(OH)2?
First, we need a balanced reaction equation
Using the volume and molarity of Ca(OH)2 the number of mol of Ca(OH)2 is
Mol of Ca(OH)2 = [0.250 mol/L] X 35.0 X 10-3 L Mol of Ca(OH)2 = 8.75 X 10-3 mol
Since there are two hydroxides in Ca(OH)2 and there is one proton in HCl, then each mol of Ca(OH)2 will react with two mol equivalents of HCl. This can be deduced from the balanced reaction equation, this is why the equation is written in the first place. A simplified form of the original equation includes only the reacting particles and excludes the spectator particles
This is called the net ionic equation.
Using the Ca(OH)2:HCl mol ratio of 1:2, the amount of HCl required to react completely with the Ca(OH)2 is
Mol of HCl = 2 X [8.75 X 10-3 mol] = 1.75 X 10-2 mol
Using the concentration of HCl = 0.375 mol/L, the volume of HCl needed to react completely with the Ca(OH)2 is
v(HCl) = 1.75 X 10-2 mol / [0.375 mol/L]
v(HCl) = 46.7 X 10-3 L = 46.7 mL
EXPERIMENTAL GUIDELINES
This is a three-part experiment,
Part I: Standardization of NaOH solution using H2C2O4 .2H2O
Part II: Standardization of NaOH solution using KHC8H4O4
Part III: Determination of the percent purity of a KHC8H4O4 sample
Each group will get a 1.0 L NaOH solution. Write your name(s) in the sticker provided and label the container. Do that as soon as you get the solution, any data that you collect applies to that one solution only. The instructions for each part are slightly different, but they all involve volumetric analysis.
The degree of success of a volumetric determination depends on how well you use the buret. In order for your buret to perform optimally, it must be properly cleaned. To clean: with the stopcock closed, add some distilled water to the buret. Tip and roll the buret, allowing the water to have contact with all of the inside surfaces. Open the stopcock and allow the water to drain. If the water drains without leaving any droplets on the side, then it is clean. After draining the final distilled water rinse, close the stopcock and add about 5 mL of the solution to be dispensed from the buret. Again, roll and tip the buret so the solution has contact with all the inside surfaces. Open the stopcock and allow the solution to drain.
Once the buret is clean, clamp it to a stand using a buret clamp. Get the appropriate amount of solution in a clean, dry beaker. Pour a few milliliters of solution into the buret. Open the stopcock all the way in order to force all the air out of the stopcock and tip. What will your results look like if the tip is empty at the beginning of the titration?
When adding solutions to the buret, make sure the stopcock is closed (horizontal position). Unclamp the buret and tilt it slightly while pouring the solution slowly down the inside surface. This will prevent the formation of air bubbles. Fill the buret to a level just above 0.00 mL. Drain the buret to just under 0.00 mL. This will properly form the meniscus. Do not try to adjust the miniscus to exactly 0.00 mL, this is a waste of time and makes your results look fake. If you want to get fuzzy with the procedure, cover the top of the buret with a loosely fitting weighting boat, piece of paper or some other material you have available. This will keep dust out of the buret.
Touch the tip of the buret to the inside wall of a beaker in order to remove any drops on the tip. Do not wipe the tip. Wait a few seconds for the solution to drain to the top of the fluid level, then record the initial buret reading in your notebook.
In order to make the meniscus easier to see, place a white card with a black mark on it behind the buret. Align the black mark so that it is just under the meniscus. Get your eye level with the bottom of the meniscus. Looking up or down on the meniscus will cause a parallax error.
The buret you will be using is the standard type...

Buret in OPEN position
since the marks occur every 0.1 mL, the buret must be read to the nearest 0.01 mL (between the marks). The last number will be an estimate.
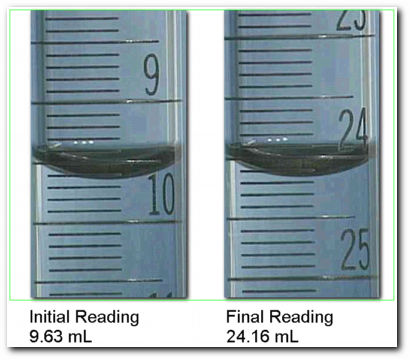
Reading the buret.
Reading 1 = 9.63 mL, Reading 2 = 24.16 mL, Volume delivered = 14.53 mL
TITRATION PROCEDURE
A known quantity of the unknown solution is pipetted into a flask (aliquot method), the unknown could also be placed directly into the flask (weight by difference method) and water added. Then a few drops of an indicator are added. Here we will use phenolphthalein, which undergoes a color change from colorless in acidic solution to pink in basic solution. Since the initial solution is acidic, it should remain colorless at this point. The flask is placed on white paper to make the endpoint easier to see.
Place the flask containing the unknown under the buret. Open the stopcock by twisting it 90 degrees into the vertical position and allow the solution to drain. As you near the desired volume, slow the flow by turning the stopcock back toward the closed position and adjust to deliver one drop at a time. You may notice a temporary color change in the solution near where the titrant was added. Stir the solution thoroughly. Any color change should disappear. As the titration progresses, the temporary color change will take longer to disappear. This signals that the endpoint is getting closer and that the titrant should be added in smaller and smaller quantities. Titrant should be added dropwise very close to the endpoint...
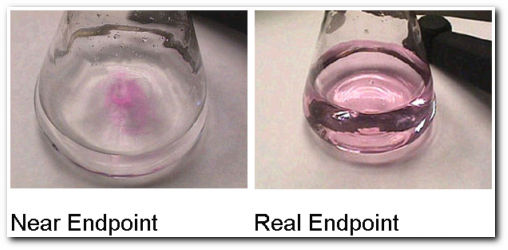
The endpoint of the titration is signaled when a permanent color change is observed (longer than 30 seconds).
It is possible to overshoot the endpoint by adding too much titrant.
A correct endpoint is shown on the left. An overshot endpoint would be darker in color.
when the desired volume has been delivered, close the stopcock. Wait a couple of seconds, then record the final buret reading. Calculate the volume delivered by subtracting the initial reading from the final reading.
When delivering solutions, you must not allow the solution to drain below the bottom of the calibration range. If this is about to occur, close the stopcock and take a final reading. Refill the buret, get an initial reading, and continue delivering solution. The total volume delivered is the sum of the volume delivered the first time and the volume delivered the second time.
PART I: STANDARDIZATION WITH H2C2O4 .2H2O
Weight out approximately 1.2-1.3 g of H2C2O4 .2H2O to the nearest mg, if the actual mass differ from 1.2 g it does not matter, what is important is that the mass reading has to be recorded accurately. Our standard procedure will be to weight by difference:
Place the solid into a 100.0 mL volumetric flask. It is best to place the sample directly into the container to avoid contamination and loss during transfer. But since the neck of a volumetric flask is very narrow you might want to use a weighting boat to collect your sample. If using a weighting boat make sure you put all of the sample into the flask, better yet, wash the boat and drain the water into the flask. Then dilute to the mark, be aware that different volumetric flasks have the 100.0 mL mark at different heights.
Compute the number of moles of H2C2O4 .H2O that you placed into the flask, using the molar mass of H2C2O4 .2H2O = 126.0 g/mol
Mol of H2C2O4 .H2O = grams of H2C2O4 .2H2O / [126.0 g/mol]
The two water molecules are there to increase the mass of the compound. Why is it bad for a solid titration standard to have a small molar mass?
When the compound goes into the solution, it falls apart...
The two waters are no longer important, at this point we will consider only the H2C2O4 part. Notice that these waters can not be ignored in the first calculation because they contribute to the molar mass of the solid.
Next you will withdraw aliquots from the volumetric flask and into an Erlenmeyer flask. If you want to get fuzzier with the procedure use different aliquot volumes. If you withdraw 20.00 mL from the volumetric flask, then you are withdrawing 20% of the total number of moles of H2C2O4, if you withdraw 23.00 mL from the volumetric flask, then you are withdrawing 23% of the total number of moles of H2C2O4 and so on. In general:
Mol of H2C2O4 withdrawn = Mol of H2C2O4 Total X [# mL withdrawn / 100 mL]
Add ~20 mL of water to the sample, this amount of water does not need to be measured accurately as it does not enter the calculations. Since H2C2O4 has two acidic hydrogens, it will react with two mol equivalent of NaOH
Then the mol equivalent of base with respect to acid is
After the titration procedure is complete, you will have determined the volume of NaOH solution that was required to achieve the end point. Using this volume and the number of mol eq of NaOH with respect to the acid procede to compute the molarity of the NaOH solution...
Mol eq of NaOH
Molarity of NaOH = ---------------------
v(NaOH) to end point
PART II: STANDARDIZATION WITH KHC8H4O4
Weight out approximately 0.40-0.50 g of KHC8H4O4 to the nearest mg using the standard procedure (weight by difference) and transfering the sample directly into an Erlenmeyer flask. Compute the number of moles of KHC8H4O4 using the molar mass of KHC8H4O4 = 204.0 g/mol
grams of KHC8H4O4
Mol of KHC8H4O4 = ----------------------
[204.0 g/mol]
Dissolve the solid in ~30-40 mL of water, this amount of water does not need to be measured accurately as it does not enter the calculations. Since KHC8H4O4 has one acidic hydrogen, it will react with one mol equivalent of NaOH
Then the mole equivalent of base with respect to acid is
After the titration procedure is complete, you will have determined the volume of NaOH solution that was required to achieve the end point. Using this volume and the number of mol eq of NaOH with respect to the acid procede to compute the molarity of the NaOH solution...
Mol eq of NaOH
Molarity of NaOH = ---------------------
v(NaOH) to end point
PART III: ANALYSIS OF KHC8H4O4 OF UNKNOWN PURITY
Weight out approximately 0.70-0.80 g of KHC8H4O4 to the nearest mg using the standard procedure (weight by difference) and transfering the sample directly into an Erlenmeyer flask. Dissolve the solid in ~30-40 mL of water, this amount of water does not need to be measured accurately as it does not enter the calculations. Since KHC8H4O4 has one acidic hydrogen, it will react with one mol equivalent of NaOH. However, the sample also contains IMPURITIES, which do not react with NaOH
IMPURITIES + KHC8H4O4 + NaOH --> NaKC8H4O4O + H2O + IMPURITIES
After the titration procedure is complete, you will have determined the volume of NaOH solution that was required to achieve the end point. Using this volume and the molarity of NaOH that you determined during the standardization procedure compute the number of moles of NaOH that were required to achieve the end point...
mol(NaOH) to end point = Molarity of NaOH X [v(NaOH) to the end point]
In the second part of the standardization procedure it was found that the mole equivalent of base with respect to acid was
Which means that
Compute the mass of KHC8H4O4 in the sample using the molar mass of KHC8H4O4 = 204.0 g/mol
Then use this value and the mass of the original sample to determine the percent purity of the sample...
% KHC8H4O4 by mass = [grams of KHC8H4O4 / grams of sample] X 100