|
|
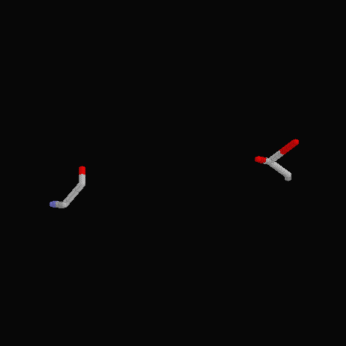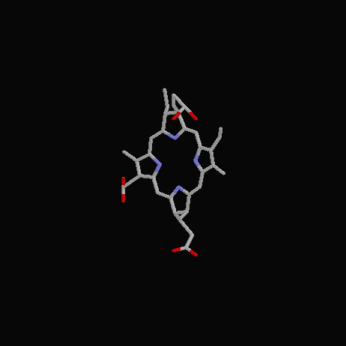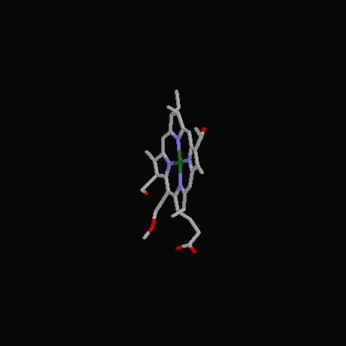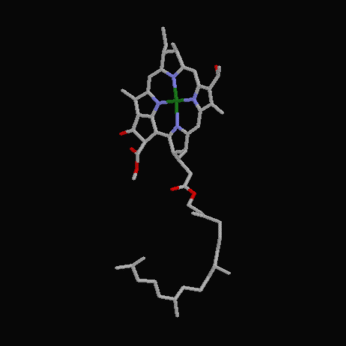 The following diagrams contain the basic steps of chlorophyll biosynthesis in static form. 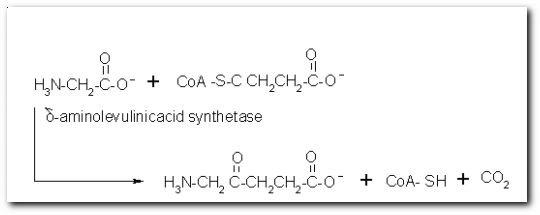 delta-aminolevunic acid is synthesized by condensation of glycine and succinyl-CoA. This reaction is catalized by the enzyme delta-aminolevunic acid synthetase. 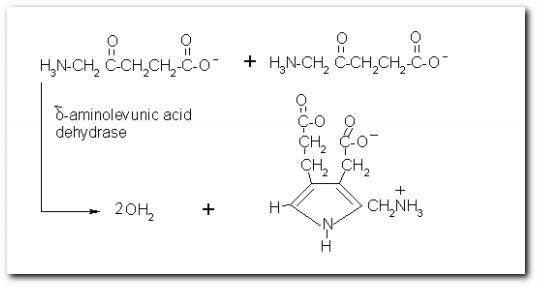 Two molecules of delta-aminolevunic acid condensate to form porphobilinogen. This is the basic pyrrole unit of the porphyrins. The raction is catalized by delta-aminolevunic acid dehydrase. 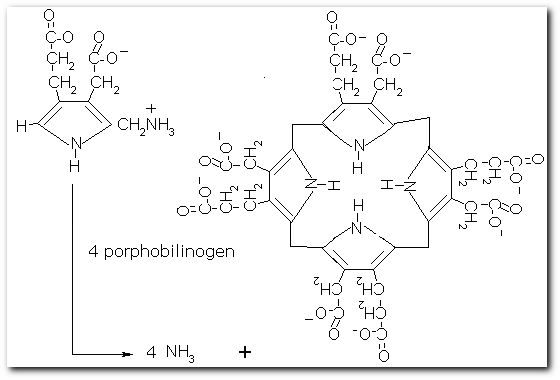 Four porphobilinogen molecules are linked into a cyclic tetrapyrrole intermediate, uroporphobilinogen I. Porphobilinogen deaminase is the enzyme that condenses the four porphobilinogen rings into uroporphobilinogen I. 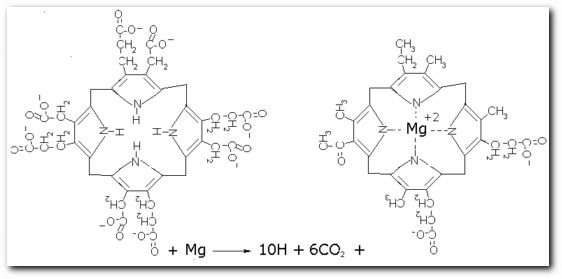 By a series of reactions, the carboxyl groups are removed, forming methyl groups. Two of the propionic acid residues are converted to vinyl groups. Further desaturation results in electronic arrangements that allow a fully conjugated system of double bonds. By chelation with Mg+2 the resulting molecule becomes the precursor of chlorophyll. 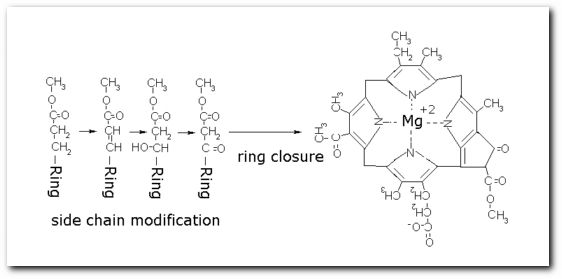 A series of side chain modifications result in the formation of the fifth ring of the structure. 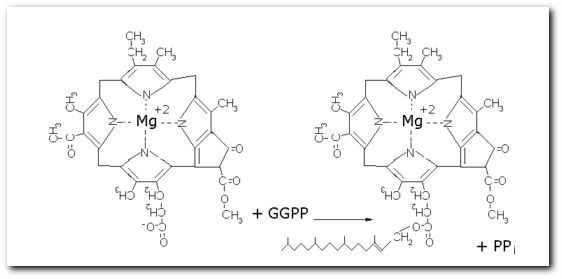 In the final step, catalized by the enzyme chlorophyll synthetase, the phosphate moiety of geranylgeranyl pyrophosphate by the porphyrin carboxyl group to form the ester linkage. Then, two of the four double bonds in the geranylgeranyl portion are reduced by NADPH to produce the phytol residue. |


