|
|
Light harvesting (LH) complexes are integral membrane proteins that form ring-like structures. Most photosynthetic bacteria contain two types of LH complexes, namely LH-I and LH-II. The LH-I complexes surround the reaction centers, so there is one LH-I complex per reaction center. The LH-IIs act as light-harvesting antennas and transfer energy to the reaction centers through the LH-I complexes. Some types of bacteria contain a third type of LH complex: the LH-III. There is no correlation between the number of reaction centers and the number of LH-II and LH-III complexes that a specific type of bacterium might contain; this characteristic is merely a function of environmental factors such as temperature and light intensity[i1]. Shown below is a live image courtesy of The Boston University pdb mirror. The heterodimer unit is part of the LH-II complex of the bacterium Rhodospirillum molischianum. Chime plug in is required to view the image.
The protein shown in the chime image is the building block of the light-harvesting complexes. When a number of them join, they form structures, 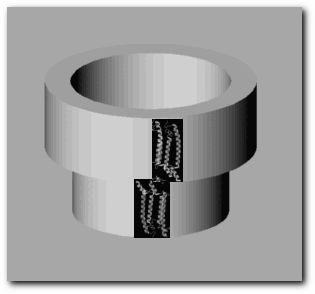 It can be seen from the picture above that the oligomer of alpha-beta heterodimers resembles two symmetric concentric rings. Pigment molecules are noncovalently bound and symmetrically arranged between these two rings. The major light-absorbing pigments in purple bacteria are bacteriochlorophyll a, bacteriochlorophyll b and carotenoids.Together,these pigments maximize the light absorbing capabilities of the LH complexes by absorbing light over a broader spectral range than the reaction centers alone[t1][t3]. LH-I complexes typically have a single near-infrared absorption band between 870 and 890 nm (with bacteriochlorophyll a) or at 1,020 nm (with bacteriochlorophyll b), slightly shifted from that of the free bacteriochlorophylls[i2]. LH-II complexes typically have two near-infrared absorption bands at 800 and 820 or 850 nm. The arrangement of the pigment molecules can be described as a series of rings. One of them is a loosely interacting ring made of a group of monomeric bacteriochlorophyll molecules. The number of bacteriochlorophylls being in a 1:1 ratio with the number heterodimer units that form the LH complex. At the opposite end of the complex there is a closely interacting ring composed of twice as many bacteriochlorophyll molecules as the other ring; these group of bacteriochlorophylls is approximately perpendicular to the first group. A third ring composed of carotenoid molecules spans the LH complex and comes into van der Waal's contact with both groups of bacteriochlorophylls. 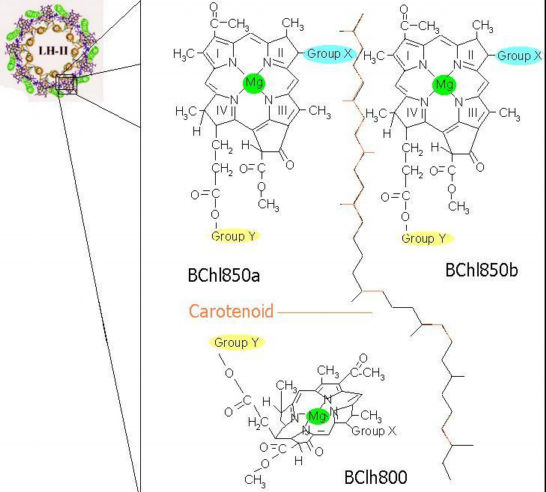 |


