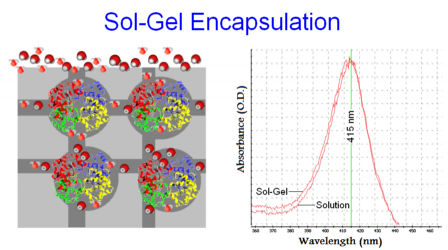
The Sol-Gel process consists of polymerization and hydrolysis of metal alkoxides like tetramethyl orthosilicate (TMOS) or tetraethyl orthosilicate (TEOS). The porous matrix allows water and small molecules to diffuse in and out. Thus when samples are submerged in buffer, the encapsulated proteins are solvated.
The UV-Vis spectra of Hemoglobin in solution and in Sol-Gel show that encapsulated Hemoglobin maintain almost intact spectral properties with respect to the solution. This means that the encapsulation procedure does not damage the protein.
Previous studies indicate that the sol-gel can be used to stabilize the protein conformation that is initially encapsulated [Uri Samuni et al J of Biol Chem., 2002, 277(28):25783-90]. An important application of this property is the encapsulation of Hemoglobin in the R- and T-state conformers by using different preparation protocols to set the initial quaternary structure to deoxygenated hemoglobin (Hb), the fully oxygenated form (HbO2), or structural states in between.

Jorge Ramos, Ph.D.
-|- SEMPER FI -|-
CURRENT DUTIES/HOBBIES
CONTACT INFORMATION
Department of Chemistry
Queens College of New York
65-30 Kissena Blvd.
Queens, NY 11367
tel: +1-718-997-3273
EMAIL:jr_starwind@netzero.net
