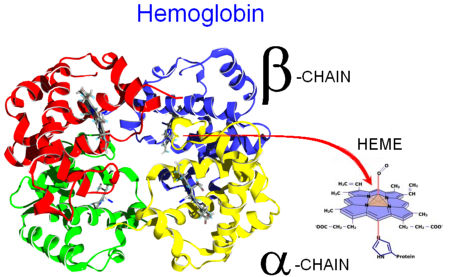
Hemoglobin is an oxygen-transport protein. An allosteric protein tetramer composed of two types of subunits designated α and β, with stoichiometry α2β2. The physiological function of Hb is derived from is ability to bind gaseous ligands such as O2, specifically at the HEME group.
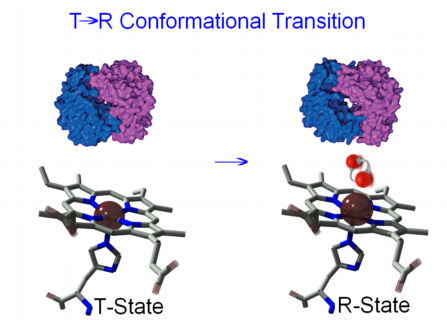
By changing its conformation, hemoglobin is able to bind and release oxygen molecules. The T → R conformational transition of hemoglobin, involving its quaternary structure is strictly related to the cooperative behavior of this protein in its reaction with oxygen. The configuration of low affinity, deoxygenated hemoglobin (Hb) is known as the tense (T) state. Conversely, the configuration of the fully oxygenated high affinity form of hemoglobin (HbO2) is known as the relaxed (R) state.
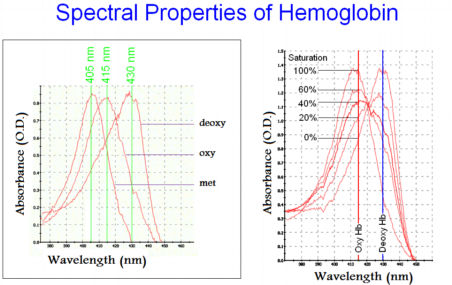
The fractional O2 saturation of Hemoglobin can be measured by recording the spectral changes of the HEME group. When the HEME bound to Hb group has its iron atom in the 3+ oxidation state (Fe3+), Hb absorbs at 405 nm, this is referred to as MetHemoglobin (MetHb). When the HEME bound to Hb group has its iron atom in the 2+ oxidation state (Fe2+), ) and there is no oxygen bound to the HEME, Hb absorbs at 430 nm, this is referred to as DeoxyHemoglobin (DeoxyHb). When DeoxyHb bind to Oxygen, Hb absorbs at 415 nm, this is referred to as OxyHemoglobin (OxyHb). The fractional O2 saturation Y can be computed from the spectral data as follows,
ODobs - OD0%
Y = --------------
OD100% - OD0%
ODobs = Absorbance of sample at 415 nm after each addition of O2
OD0% = Absorbance of DeoxyHb at 415 nm (0% saturation)
OD100% = Absorbance of OxyHb at 415 nm (100% saturation)

Jorge Ramos, Ph.D.
-|- SEMPER FI -|-
CURRENT DUTIES/HOBBIES
CONTACT INFORMATION
Department of Chemistry
Queens College of New York
65-30 Kissena Blvd.
Queens, NY 11367
tel: +1-718-997-3273
EMAIL:jr_starwind@netzero.net
